Breathing Beyond Barriers: The Art, Science, and Surprises of NIV & LTOT in Obstructive Airway Diseases
Years ago, in a dim ICU, I witnessed a patient turn the tide of acute respiratory failure with an oronasal NIV interface — a vignette forever shaping my practice and curiosity. Since then, the evolution from primitive positive airways to today's nuanced, evidence-backed NIV and LTOT interventions remains nothing short of remarkable — and occasionally, confounding. What really matters in modern practice: the guideline, the device, the patient's story, or the artful judgment of the clinician? Let's breathe new life into this conversation, challenge dogma, and bridge gaps between science, devices, and real human decisions.
Clinical Applications: Where Science Meets Bedside (and Sometimes Fails)
When I think about the clinical applications of noninvasive ventilation (NIV) and long-term oxygen therapy (LTOT) in obstructive airway diseases, I’m reminded that the intersection of science and bedside medicine is rarely straightforward. The evidence is robust in places, murky in others, and sometimes, our instincts as clinicians are challenged by what the data actually show. Let’s walk through how clinical applications of NIV and LTOT play out in real-world practice, guided by evidence-based recommendations—and a few surprises along the way.
Acute vs. Chronic: The Two Faces of NIV
First, it’s crucial to distinguish between acute respiratory failure and chronic, stable disease. In the acute setting—think COPD exacerbations, hypercapnic crises, or post-extubation in high-risk patients—NIV is a game-changer. Guidelines strongly recommend NIV for acute hypercapnic respiratory failure, especially in immunosuppressed patients or those at high risk for recurrent failure after extubation. Here, the numbers speak volumes: research shows mortality reductions of up to 50% in select groups, and intubation avoidance rates ranging from 30% to 80%, depending on patient selection and underlying etiology.
But not all patients benefit equally. For those at low risk of respiratory failure post-extubation, the evidence is less compelling. In these scenarios, the risks of unnecessary intervention may outweigh the benefits. This is where clinical judgment—sometimes at odds with our urge to “do something”—becomes paramount.
Chronic Use: Timing and Patient Selection Matter
When it comes to long-term NIV in chronic stable hypercapnic COPD, the story gets more nuanced. Nocturnal NIV is suggested for patients with persistent hypercapnia, but only after screening for comorbid obstructive sleep apnea (OSA). Why? Because OSA can confound both the diagnosis and the response to therapy. The timing of initiation is also critical: long-term NIV should not be started during an acute episode. Instead, guidelines recommend reassessment 2–4 weeks after the acute event has resolved, ensuring that only those with persistent hypercapnia are selected for ongoing therapy.
Outcomes That Shape Practice
So, what outcomes drive these recommendations? Three stand out:
- Mortality outcomes—the gold standard for any intervention.
- Rates of intubation avoidance—a key marker of NIV’s success in acute settings.
- Nosocomial pneumonia—significantly lower with NIV (6–8%) compared to invasive ventilation (20%) in some cohorts.
These outcomes aren’t just numbers; they’re the backbone of evidence-based recommendations and the reason why guideline panels prioritize them when shaping clinical practice.
When Instincts Clash with Evidence: A Bedside Anecdote
I’ll never forget a case early in my career: an elderly COPD patient, post-extubation, seemed stable. My instinct was to “watch and wait,” but the latest guidelines nudged me toward early NIV. The team debated—intuition versus evidence. We started NIV, and the patient avoided reintubation, walking out of the ICU days later. It was a humbling reminder that, as Dr. Claudia Craven puts it:
“Innovation in respiratory support fails without astute clinical judgment — numbers can never replace intuition.”
Comparing Outcomes: NIV vs. Invasive Ventilation
| Outcome | NIV | Invasive Ventilation |
|---|---|---|
| Mortality Reduction (Acute RF) | Up to 50% (select groups) | Baseline |
| Intubation Avoidance | 30–80% | N/A |
| Nosocomial Pneumonia | 6–8% | 20% |
| Reassessment for LT NIV | 2–4 weeks post-acute | N/A |
Visualizing the Evidence
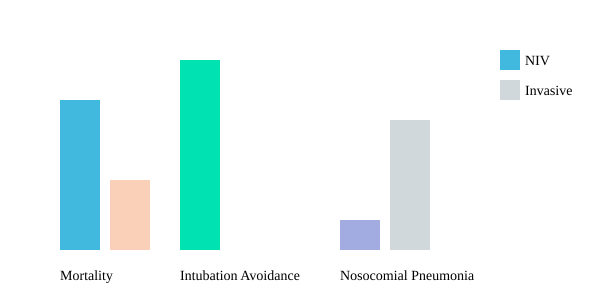
Ultimately, the art and science of clinical applications of NIV demand a balance—between evidence, patient context, and the wisdom that comes only from experience.
Selection Criteria: Navigating the Gray Zones (And the Exceptions That Prove the Rule)
When it comes to NIV patient selection and LTOT strategies in obstructive airway diseases, the science is robust—but the art is in the details. As a pulmonologist, I’ve learned that the guidelines provide a framework, but the real-world application is often messier. Let’s break down what truly matters when choosing the right patients for noninvasive ventilation (NIV) or long-term oxygen therapy (LTOT), and how to approach those inevitable gray zones that every clinician faces.
Clinical and Physiological Criteria: Where Evidence Meets Practice
The foundation of NIV patient selection is evidence-based. Research shows that the recommended arterial pH threshold for NIV initiation in acute COPD exacerbations sits between 7.30 and 7.35. This isn’t arbitrary—patients below this range are at higher risk for rapid deterioration, while those above may not benefit as much from NIV. Hypercapnia, particularly evolving or persistent elevations in PaCO2, is another cornerstone. But it’s never just about the numbers.
Guideline committees emphasize that comorbidities, especially obstructive sleep apnea (OSA), must be screened for before starting chronic nocturnal NIV in COPD. Studies indicate that OSA is present in roughly 25% of chronic hypercapnic COPD patients, and missing this can undermine the effectiveness of both NIV and LTOT strategies.
Psychosocial and Practical Issues: The Human Factor
Patient-reported dyspnea is a key driver for therapy, but psychosocial dynamics can’t be ignored. I’ve seen patients who meet every clinical criterion but simply cannot tolerate the mask. In fact, intolerance rates to NIV interfaces can reach up to 20%, depending on the interface and patient factors. Anxiety, claustrophobia, and even social support at home play a major role in adherence.
As Dr. Maria Yanez wisely put it:
"Sometimes, the difference between NIV success and failure is one midnight conversation about claustrophobia."
This quote rings true in practice. Interpersonal skills—negotiation, empathy, adaptability—are as critical as any algorithm. There are no shortcuts here.
Gray Zones: Borderline pH, Fluctuating Hypercapnia, and Tolerance Dilemmas
Let’s talk about the gray areas. What do you do with a patient whose pH hovers at 7.34, or whose hypercapnia fluctuates day to day? The answer is rarely black and white. I often find myself weighing the risks and benefits, considering comorbidities, and—crucially—re-evaluating after the acute event. The guideline committee recommends a 2–4 week re-evaluation period post-acute event before initiating long-term NIV. This window allows for stabilization and a more accurate assessment of ongoing need.
What-If Scenario: The Anxious COPD Patient
Imagine a patient with severe COPD, clear hypercapnia, but overwhelming anxiety about the mask. Do you persist, or do you pull back? In my experience, I start with education, trial different interfaces, and involve family or psychological support. If, after all efforts, the patient remains intolerant, it may be safer to escalate care or consider alternative strategies. Adverse events or poor tolerance are not failures—they’re signals to re-evaluate.
Key Data Table: Selection Criteria and Barriers
| Selection Factor | Key Data/Threshold |
|---|---|
| pH threshold for NIV initiation | 7.30–7.35 (acute COPD) |
| NIV interface intolerance rate | Up to 20% |
| OSA prevalence in chronic hypercapnic COPD | ~25% |
| Re-evaluation period post-acute event | 2–4 weeks |
Selection Barriers Among NIV/LTOT Candidates
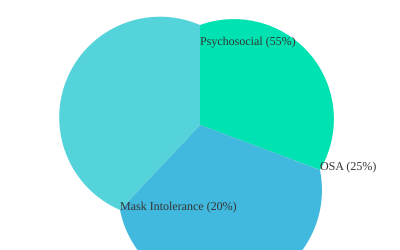
This pie chart illustrates the relative proportions of common selection barriers among NIV/LTOT candidates: OSA presence, mask intolerance, and psychosocial factors. Each slice is a reminder that practical issues are as real as any lab value.
Practical Implementation: Devices, Interfaces, and The Fine Art of Making It Work
When it comes to noninvasive ventilation (NIV) in obstructive airway diseases, the real magic happens not just in the science, but in the subtle art of practical implementation. Over the years, I’ve learned that choosing the right device and interface is rarely a one-size-fits-all equation. Instead, it’s a dynamic process—balancing evidence, patient anatomy, and a healthy dose of clinical intuition. As Dr. Jennifer Pace puts it:
“There’s as much art as algorithm in picking the right NIV setup for the right patient — sometimes, it’s the little things that matter most.”
NIV Interface Selection: Nasal, Oronasal, and Full-Face Realities
Let’s start with the basics: NIV interface selection is foundational. The main options—nasal interfaces, oronasal interfaces, and full-face masks—each come with their own quirks and benefits. Research shows that nasal interfaces are preferred in about 40% of COPD cases. They’re lightweight and less claustrophobic, but can be tricky for mouth breathers or those with severe nasal congestion. Oronasal interfaces, on the other hand, offer a more secure seal and are often better tolerated by patients who struggle to keep their mouths closed during sleep. Full-face masks are reserved for those who need maximum support, but they can increase the risk of skin breakdown and pressure sores.
In my experience, there’s no universal “best” interface. Fit and patient preference drive decisions. Sometimes, it takes a few swaps to find the right match. I’ve seen patients thrive with a nasal mask one week, only to switch to an oronasal interface after developing nasal dryness or leaks. The key is flexibility—and a well-stocked interface cart.
Device Choices: Bilevel Devices vs. Continuous Positive Airway Pressure (CPAP)
Device selection is another critical step. Bilevel devices are the first line for most COPD patients. They provide separate inspiratory and expiratory pressures, which helps reduce the work of breathing and improve ventilation. CPAP, or continuous positive airway pressure, is typically reserved for those with an obstructive sleep apnea (OSA) overlap. I recall a workshop where a patient with OSA-predominant overlap was mistakenly started on a bilevel device. The result? Alarms blaring, patient discomfort, and a quick lesson in the importance of matching device to diagnosis.
Maintenance Musts: Keeping It Clean and Functional
Maintenance protocols can make or break NIV success. Regular filter changes, daily cleaning of interfaces, and prompt troubleshooting of alarms are non-negotiable. Studies indicate that mask-related complications—like leaks, pressure sores, and skin breakdown—lead to interface changes in about 15% of cases. In busy units, alarms can trigger 10-15 nurse call-ins per night, often due to mask leaks or patient movement. Staying ahead of these issues means being proactive: rotate interfaces, inspect skin daily, and educate staff on alarm management.
Wild Card Wisdom: DIY Comfort Hacks from the Front Lines
Sometimes, it’s the little tricks that make the biggest difference. Veteran clinicians have shared simple hacks—like using a soft cloth or hydrocolloid dressing under mask edges to prevent pressure sores, or gently warming humidified air to reduce nasal dryness. These aren’t in the textbooks, but they’re gold on the ward.
| Data Point | Value |
|---|---|
| Nasal interface preference in COPD | ~40% |
| Mask-related complication rates (interface change) | ~15% |
| Bilevel devices (first line in COPD) | Yes |
| CPAP (reserved for OSA-predominant overlap) | Yes |
| Alarms triggering nurse call-ins (per night) | 10-15 |
Interface Usage in Clinical NIV for COPD
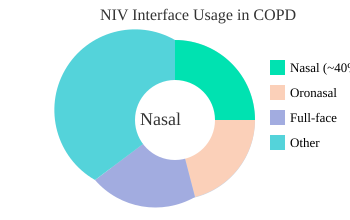
Ultimately, practical implementation of NIV is a blend of science, skill, and a willingness to adapt. The best outcomes come from staying flexible, listening to patients, and never underestimating the power of a well-chosen interface or a timely comfort hack.
Guideline Evolution: From Ivory Towers to Bedside Realities
When I first started working with official ERS/ATS guidelines, I was struck by how much the process of guideline development had evolved. Gone are the days when a handful of experts would gather in a closed room and emerge with a set of recommendations. Today, the creation of clinical guidelines—especially those shaping the use of Non-Invasive Ventilation (NIV) and Long-Term Oxygen Therapy (LTOT) in obstructive airway diseases—is a rigorous, transparent, and multidisciplinary endeavor.
Who Sits at the Table? Committee Composition and Dynamics
Guideline panels typically include 10–25 members, with representation from at least three different disciplines. In my experience, these panels bring together pulmonologists, intensivists, respiratory therapists, and methodologists. This diversity ensures that recommendations reflect a broad spectrum of clinical realities, not just academic ideals. The committee composition directly influences which outcomes are prioritized—whether it’s mortality, hospital readmission, or patient-reported quality of life.
The process is also shaped by the inclusion of patient advocates and, increasingly, health economists. Their input helps ensure that guidelines are not only evidence-based but also practical and patient-centered.
Methodological Advances: GRADE and PICO Lead the Way
One of the most significant advances in guideline development has been the adoption of the GRADE methodology. Research shows that over 80% of new respiratory guidelines now use GRADE to evaluate the certainty of evidence and the strength of recommendations. This approach brings a level of transparency and reproducibility that was previously lacking.
Alongside GRADE, the PICO format (Population, Intervention, Comparator, Outcome) has become the standard for structuring clinical questions since around 2010. This framework ensures that each recommendation is anchored in a clear, answerable question, making the resulting technical summaries and narrative literature reviews more accessible and actionable for clinicians.
Setting Priorities: Outcomes That Matter
A memorable moment from a recent committee meeting comes to mind. We spent nearly an hour debating whether “hospital-free days” or “all-cause mortality” should be the primary outcome for a new NIV guideline. It was a passionate, sometimes circular discussion, but it highlighted how the outcomes prioritized can shape not just the recommendations, but also how they’re interpreted at the bedside. Ultimately, the consensus leaned toward patient-centered outcomes, reflecting a broader shift in the field.
Transparency and Conflict of Interest Management
Transparency has become a cornerstone of modern guideline development. Since 2015, conflict of interest disclosures have been required in 100% of official guidelines. This is more than a bureaucratic formality—it’s about maintaining trust and ensuring that recommendations are free from undue influence. Confidentiality agreements and public posting of disclosures are now standard practice.
Dr. Rakesh Jain: "The best guidelines don’t dictate — they inform, inspire, and adapt to the messiness of real medicine."
Guideline Development at a Glance
| Aspect | Details |
|---|---|
| Panel Size | 10–25 members |
| Disciplines Represented | At least 3 (e.g., pulmonology, critical care, methodology) |
| Conflict of Interest Disclosures | Required in 100% of guidelines since 2015 |
| GRADE Methodology Use | >80% of new respiratory guidelines |
| PICO Format Adoption | Standard since ~2010 |
Visualizing the Process: From Evidence to Consensus
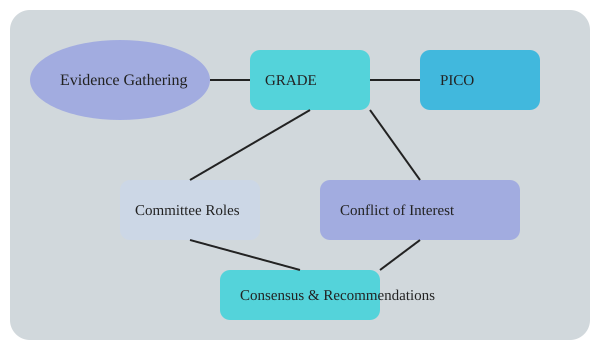
As we can see, the evolution of official ERS/ATS guidelines is not just about following a checklist. It’s a living process—one that blends art, science, and the realities of clinical practice, all while striving for transparency and patient-centered care.
The Next Frontier: Innovations, Unanswered Questions, and the Human Element
As I reflect on the evolving landscape of NIV application and LTOT in obstructive airway diseases, it’s clear that we are standing at a crossroads of technology and tradition. The past decade has brought remarkable advances—AI-driven ventilation algorithms, remote adherence monitoring, and patient-centric interface design are no longer just buzzwords, but realities shaping our daily practice. These innovations are not only enhancing the precision of care but are also challenging us to rethink the boundaries of what’s possible in respiratory medicine.
Breakthroughs in Technology: More Than Just Gadgets
Let’s start with what’s new. Remote monitoring systems for LTOT have demonstrated a 15-25% improvement in compliance rates in pilot studies, a leap that can’t be ignored. Algorithm-based ventilator tuning, meanwhile, has reduced asynchrony events by 30% in observational trials. These are not just incremental tweaks—they represent a paradigm shift in how we deliver and monitor therapy. Smart interfaces are making the patient experience more intuitive, and data transmission is now seamless, allowing for real-time adjustments and more personalized care.
| Innovation | Impact |
|---|---|
| Remote adherence monitoring (LTOT) | 15-25% improved compliance (pilot studies) |
| Algorithm-based ventilator tuning | 30% reduction in asynchrony events (observational trials) |
| PROM uptake in trials | Up to 35% |
| Palliative NIV clinical trials | <5% of total NIV research |
Unanswered Questions: Evidence Gaps and the Human Experience
Yet, for all this progress, there are stubborn gaps. Research shows that randomized controlled trials (RCTs) remain limited, especially in frail, multimorbid populations—the very patients who may benefit most from nuanced clinical practice guidelines and tailored interventions. Patient-reported outcome measures (PROMs), such as subjective dyspnea, are underutilized, with uptake in trials still hovering at just 35%. And when it comes to palliative care, the evidence base is even thinner: less than 5% of NIV research focuses on end-of-life applications, despite growing clinical need.
This is where supplemental material and ongoing research become invaluable. We need more robust data, especially around the use of NIV and LTOT in advanced disease stages, and a deeper understanding of how patients perceive their own symptoms and quality of life. The nuances of palliative care—balancing symptom relief, dignity, and family wishes—demand more than algorithms can currently provide.
The Human Element: Where Guidelines, Technology, and Intuition Meet
Despite all the technological promise, I’m reminded daily that the art of medicine is never fully automated. As Dr. Priya Sharma wisely puts it:
"Technology may dazzle, but the patient’s sigh, smile, or struggle still tells you more than any data feed."
This rings especially true in the context of evidence-based recommendations. While guidelines and digital tools are essential, our clinical judgment—shaped by empathy, presence, and years of bedside experience—remains irreplaceable. Sometimes, the right decision emerges not from a protocol, but from a quiet conversation at the bedside, or a subtle change in a patient’s demeanor.
Looking Ahead: A Future Scenario
Imagine a patient with advanced COPD, monitored remotely, their ventilator settings adjusted by AI in real time. Their PROMs are logged seamlessly, and alerts ping the care team if distress is detected. Yet, in a moment of crisis, it’s the clinician’s intuition—perhaps a gentle hand on the shoulder or a decision to pause and listen—that makes all the difference. As we embrace the next frontier of NIV and LTOT, let’s champion both innovation and the irreplaceable human touch.
TL;DR: NIV and LTOT, when applied with attention to evidence and the realities of practice, can transform outcomes in obstructive airway diseases—but personalization, vigilance, and reflective curiosity remain essential amid evolving guidelines and technology.
Comments
Post a Comment