Beyond One-Size-Fits-All: How COPD Phenotyping Revolutionizes Patient Care (and Why It Matters More Than You Think)
I’ll never forget the first time I realized my COPD patients weren’t a monolithic crowd, despite what my old textbooks suggested. Two men, same age and smoking history, but one was back in the ER every month while the other barely seemed winded. That’s the moment phenotype-based care made sense to me – and from that day, my approach to COPD management was forever changed. In a world where ‘average’ rarely means much for an individual, phenotyping gives us a shot at true personalized medicine. Let’s unravel what this really means for people with COPD (and those treating them) through stories, surprising numbers, and a few lessons learned.
The Problem with ‘One Size Fits All’ in COPD Care – and My Early Wake-up Call
For years, COPD management was built on a simple idea: measure lung function (usually FEV1), assign a stage, and treat everyone in that category the same. It seemed logical, even elegant. But as I learned early in my career, this “one-size-fits-all” approach to COPD care can be dangerously misleading. Let me share why—and how understanding COPD phenotypes has completely changed the way I practice.
When Spirometry Isn’t the Whole Story
I remember two patients from my first year in pulmonary medicine. Both had nearly identical spirometry scores—moderate airflow limitation, textbook FEV1 values. Yet, their lives couldn’t have been more different. One was in and out of the hospital every few weeks, struggling with breathlessness and anxiety. The other managed daily walks and rarely needed urgent care. If I’d relied on lung function alone, I would have missed the real drivers of their disease.
Research shows that ignoring phenotypic differences in COPD can lead to poor care and missed opportunities. Recent studies indicate that patient outcomes—like survival rates and quality of life—often hinge on subtle clinical nuances, not just test results. This is where personalized medicine and COPD risk stratification come into play.
Phenotypes: The Key to Personalized COPD Management
COPD is not a single disease, but a collection of overlapping syndromes. Phenotyping allows us to group patients by shared characteristics that actually matter for prognosis and treatment. For example:
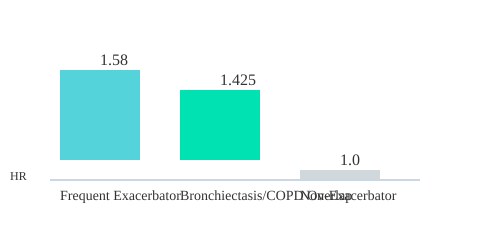
- Frequent exacerbators face a 58% higher mortality risk (hazard ratio [HR] 1.58).
- Bronchiectasis/COPD overlap increases mortality risk by 42.5% (HR 1.425).
- Non-exacerbators—about two-thirds of patients, per the POPE study—have much lower risk profiles.
These differences aren’t just academic. They drive real-world decisions: who needs inhaled corticosteroids, who might benefit from lung volume reduction surgery, and who requires early referral for transplant evaluation. Textbook FEV1 doesn’t always predict who will end up in the hospital next month.
Why Phenotyping Matters (and My Wake-up Call)
I once cared for a patient labeled “non-compliant” after repeated ER visits. On closer review, detailed phenotyping revealed upper-lobe emphysema and a strong family history of atopy. This changed everything—her management shifted from generic protocols to targeted therapies, and her hospitalizations dropped dramatically. It was a powerful lesson: COPD phenotypes unlock better risk stratification and management than generic GOLD staging.
'Treating every COPD patient the same is like giving every baker the same recipe – some get perfect bread, others burnt crust.' – Dr. Ana Gutierrez
Today, we know that frequent exacerbator and emphysematous patients have dramatically different hospital and therapy needs. Younger “rapid decliners” may slip through the cracks without tailored tools. Personalized medicine isn’t just a buzzword—it’s the difference between missed chances and meaningful care.
Comparing Mortality Risk by COPD Phenotype
| Phenotype | Hazard Ratio (HR) | Population Prevalence |
|---|---|---|
| Frequent Exacerbator | 1.58 | ~33% |
| Bronchiectasis/COPD Overlap | 1.425 | Variable |
| Non-Exacerbator | Reference | ~67% (POPE Study) |
Mortality Hazard Ratios by COPD Phenotype
The shift to COPD phenotyping is more than a trend—it’s a revolution in COPD management and risk stratification, offering hope for truly personalized medicine.

Mapping the Key COPD Phenotypes: Why Labels Matter (More Than You Think)
When I first started learning about COPD phenotypes, I’ll admit—I wondered if these labels were just academic. But the more I dug into the research, the clearer it became: these labels aren’t just for show. They’re the backbone of modern COPD mortality risk stratification and the key to truly personalized care.
Let’s break down the main COPD phenotypes that are reshaping how we treat and predict outcomes for patients:
- Asthma-COPD Overlap
- Frequent Exacerbator
- Upper Lobe-Predominant Emphysema
- Cachectic Phenotype
- Rapid Decliner
- Physical Frailty
- Comorbid Phenotypes
Each of these phenotypes has specific implications for therapy, risk, and prognosis. For example, the Frequent Exacerbator phenotype is linked to a 58% higher mortality risk (HR 1.58), and these patients benefit from aggressive inhaled corticosteroids and bronchodilators. Meanwhile, those with Upper Lobe-Predominant Emphysema may be candidates for surgical lung volume reduction, which can dramatically improve quality of life.
But here’s where it gets even more interesting—and challenging. Patients rarely fit neatly into one box. Overlapping phenotypes are common, and the risks add up. Research shows that a patient with both emphysema and cachexia who also experiences frequent exacerbations faces a hazard ratio of 3.075. That’s a 5-year mortality risk of 77.6%. The sum, in this case, is deadlier than the parts.
| Phenotype | Hazard Ratio (HR) | 5-Year Mortality (%) |
|---|---|---|
| Cachexia | 2.262 | — |
| Emphysematous | 1.786 | — |
| Overlap (Emphysema + Cachexia + Exacerbator) | 3.075 | 77.6 |
The POPE study, which looked at over 3,300 patients across Central and Eastern Europe, found that about two-thirds were non-exacerbators. But the rest? Their phenotype distribution varied by country, highlighting the importance of geographic variations in COPD phenotypes. This means that what works for one population may not be best for another—a crucial insight for clinicians working in diverse settings.
The Spanish Society of Pulmonology and Thoracic Surgery was the first to make phenotype-based treatment the standard back in 2012. Since then, the field has moved toward a “treatable traits” approach, recognizing that patients often display multiple, overlapping characteristics. This shift helps us move beyond rigid categories and focus on what’s actually actionable for each patient.
Why does all this matter? Because recognizing and acting on these phenotypes allows us to:
- Choose therapies that target the underlying disease process
- Identify high-risk patients early for intensive monitoring
- Optimize resource allocation and referral timing
- Personalize follow-up based on real risk—not just spirometry
'The power of phenotyping lies in seeing the hidden patterns that define a patient's future.' – Prof. Francisco Rodriguez-Roisin
Ultimately, understanding COPD phenotypes isn’t just about labels. It’s about seeing the patient in front of us—complex, unique, and deserving of care that matches their individual risk and needs.
Phenotype-Specific Therapies in Action: More Than Just a Buzzword
When I first started managing COPD, I leaned heavily on the “one-size-fits-all” approach—bronchodilators for everyone, maybe inhaled corticosteroids (ICS) if things got rough. But as research shows, this strategy misses the mark for many patients. Today, phenotype-specific therapies are changing the game, allowing us to deliver tailored interventions for COPD that truly make a difference.
Why Phenotyping Matters in COPD
COPD is not a single disease—it’s a spectrum, with each patient presenting unique patterns and risks. By identifying phenotypes, we move beyond basic spirometry and start matching the right treatment to the right patient. Studies indicate that this approach not only improves outcomes but also optimizes resource use and reduces unnecessary side effects[1][2].
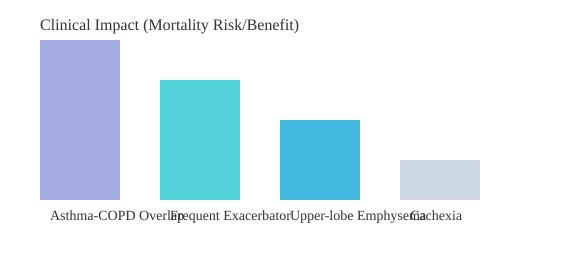
Asthma-COPD Overlap: ICS Is Essential
For patients with asthma-COPD overlap, inhaled corticosteroids are fundamental, not optional. These individuals often have a history of atopy or asthma and show a better response to ICS than other COPD subtypes. I’ve seen patients stabilize dramatically once ICS is prioritized—something that’s backed by strong evidence and now considered standard of care.
Frequent Exacerbators: Aggressive, Multi-Modal Therapy
Frequent exacerbators—those who suffer repeated flare-ups—face a threefold increase in mortality risk. Here, long-acting bronchodilators and ICS are just the start. We often add macrolides, roflumilast, or acetyl-cysteine for their anti-inflammatory effects. Research supports this aggressive approach, showing reduced hospitalizations and improved survival[1][4].
Upper-Lobe Emphysema: Surgical Solutions
Upper-lobe-predominant emphysema is a distinct phenotype where lung volume reduction surgery (LVRS) can be life-changing. I’ll never forget a patient who, after LVRS, went from housebound to walking daily—challenging my own assumptions about “end-stage” disease. This intervention is often overlooked without proper phenotyping, yet it offers the greatest benefit to this subgroup[3].
Cachexia: More Than Just Weight Loss
Cachexia in COPD signals a 126% higher mortality risk. These patients need more than inhalers—they require nutritional support, metabolic monitoring, and sometimes systemic therapy. It’s a reminder that phenotype-specific therapies must address the whole patient, not just their lungs.
Rapid Decliners and Comorbid/Frailty Phenotypes
Some patients, especially younger ones, experience rapid lung function decline. Early referral for transplant evaluation can be lifesaving. Others present with significant comorbidities or frailty, where integrated rehabilitation and cardiovascular/metabolic management take precedence over respiratory meds alone. This is where tailored interventions for COPD truly shine.
'Treating the phenotype isn't a luxury—it's a necessity.' – Dr. Silvia Martinez
Table: Phenotype-Specific Interventions and Clinical Impact
| Phenotype | Key Intervention | Clinical Impact |
|---|---|---|
| Asthma-COPD Overlap | ICS indicated | Improved control, reduced exacerbations |
| Frequent Exacerbator | Long-acting bronchodilators, ICS, macrolides, roflumilast | 3x higher mortality risk if untreated |
| Upper-lobe Emphysema | LVRS | Best response, improved quality of life |
| Cachexia | Nutrition support, metabolic therapy | 126% higher mortality risk if unmanaged |
Chart: Clinical Impact of Phenotype-Specific Therapies
The evolution toward precision medicine in COPD is not just a trend—it’s a necessity. By recognizing and treating phenotypes, we’re not only improving outcomes but also redefining what’s possible for our patients.

Additive Risk Isn’t Just Academic—Here’s What It Actually Looks Like Clinically
When we talk about COPD phenotypes clinical significance, it’s easy to imagine neat, separate boxes: one patient with emphysema, another with cachexia, a third who’s a frequent exacerbator. But in real-world practice, these lines blur. Many patients rack up multiple phenotypes—think emphysema and cachexia and frequent exacerbations. Each added phenotype doesn’t just nudge up the risk; it stacks the odds against long-term survival in a way that’s both dramatic and sobering.
Research shows that the overlap of these phenotypes creates a risk profile far more severe than any single trait alone. For instance, a patient with all three—emphysema, cachexia, and frequent exacerbator status—faces a hazard ratio (HR) of 3.075 for mortality. That translates to a five-year mortality rate approaching 78%. To put it plainly: nearly four out of five of these patients will not survive five years, even if they’re relatively young and otherwise free of major comorbidities.
| Phenotype Combination | Hazard Ratio (HR) | Five-Year Mortality (%) |
|---|---|---|
| Emphysema + Cachexia + Frequent Exacerbator | 3.075 | 77.6 |
This is where COPD risk stratification and COPD mortality risk stratification become more than academic exercises. In my own practice, I recall a middle-aged patient who checked all three boxes. Despite not fitting the “classic” advanced COPD profile by age or spirometry alone, his risk was off the charts. Our team moved quickly—early referral for transplant evaluation, more aggressive pharmacologic therapy, and a multidisciplinary approach to nutrition and rehabilitation. Standard COPD treatment algorithms would have missed the urgency, focusing only on FEV1 or exacerbation count.
Guideline algorithms that ignore phenotype overlap can miss critical windows for early intervention. As studies indicate, phenotype overlap raises risk far beyond the sum of single traits. This means some patients will need evaluation for transplantation or advanced therapies far sooner than age or spirometry would suggest. The additive risk is not just a number—it’s a call to action for integrated, proactive care.
Let’s break it down further. Each phenotype brings its own management implications:
- Emphysema: May benefit from lung volume reduction strategies and targeted bronchodilation.
- Cachexia: Requires nutritional intervention and metabolic support.
- Frequent Exacerbator: Needs intensified anti-inflammatory therapy and close monitoring to prevent hospitalizations.
But when these phenotypes overlap, the management plan must be even more aggressive and coordinated. Early, specialized intervention is not optional—it’s essential. As Dr. Petra Novak puts it:
“Recognizing superimposed phenotypes transformed how our multidisciplinary team plans follow-up.”
Incorporating structured phenotyping into routine assessment isn’t just about ticking boxes. It’s about seeing the whole patient and anticipating the trajectory of their disease. The data is clear: additive mortality risk from overlapping phenotypes demands a shift from generic protocols to individualized, proactive care. This is the heart of COPD phenotypes clinical significance—and why it matters more than you might think.
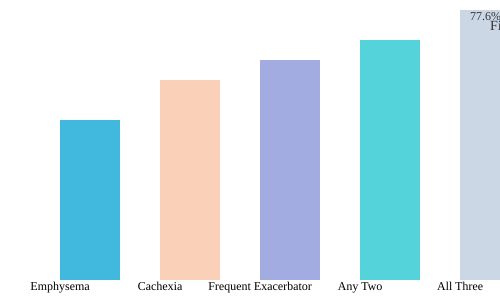
Screening Tools and Real-World Challenges: Moving from Theory to Practical Care
When we talk about COPD phenotyping screening tools, it’s tempting to imagine a seamless process—identify the phenotype, match the treatment, and watch outcomes improve. But, as I’ve seen firsthand, the reality is much messier. The journey from theory to practical care is full of hurdles, and the tools we use to screen and classify patients play a crucial role in whether we catch high-risk individuals early or miss them entirely.
Why Validated Screening Tools Matter
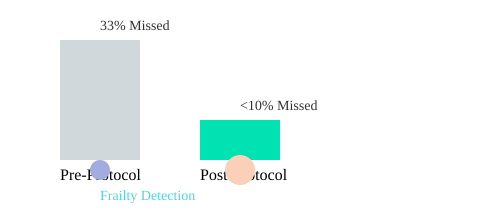
Research shows that validated tools like the 4-meter gait speed test and standardized depression screens are game-changers for detecting frailty and psychiatric comorbidities in COPD. These aren’t just academic exercises—they directly impact how we identify patients who might otherwise slip through the cracks. For example, frailty is a powerful predictor of poor outcomes, yet it often goes unnoticed unless we actively look for it.
In my own clinic, we conducted an audit before adopting a structured phenotyping protocol. The results were sobering: 1 in 3 high-risk patients had significant comorbid risk factors that were missed. After implementing a standardized assessment protocol, our detection rates for frailty and other comorbidities improved dramatically.
| Phase | High-Risk Patients Missed | Frailty Identification Rate |
|---|---|---|
| Pre-Protocol | 33% | Low |
| Post-Protocol | <10% | Significantly Higher |
SVG Chart: Frailty/Comorbid Detection Pre- and Post-Protocol
Standardized Protocols: The Key to Early Intervention
This is where early intervention strategies for COPD truly shine. By embedding validated screening tools into routine care, we can spot treatable traits—like frailty, depression, or frequent exacerbations—before they spiral into hospitalizations or rapid decline. It’s not just about ticking boxes; it’s about changing the trajectory of the disease for each patient.
But let’s be honest: front-line implementation is inconsistent. Even with the best intentions, busy clinics often default to the basics, and nuanced comorbidities get overlooked. That’s why comprehensive protocol development is so important. It’s about building a system that doesn’t rely on memory or luck to catch the high-risk cases.
From Phenotypes to Treatable Traits
The field is evolving. While phenotype-based management—like identifying frequent exacerbators or asthma-COPD overlap—has improved COPD comorbidities management, we’re now moving toward a treatable traits approach. Instead of rigid labels, we focus on specific, modifiable targets: is the patient frail? Depressed? Prone to exacerbations? Each trait becomes a doorway to personalized treatment.
'You can’t fix what you don’t measure – phenotyping only works when backed by solid screening.' – Dr. Leah Kim
Complexity in the Real World
Of course, not every patient fits neatly into a single box. Overlapping or atypical presentations are common, and that’s where clinical judgment and flexible protocols matter most. Still, the evidence is clear: consistent, validated screening is the foundation for unlocking the full benefit of phenotype-driven care. The next step? Making sure every patient actually gets it.

Geography, Guidelines, and the Ongoing Evolution Toward Precision Medicine
When we talk about COPD phenotypes, it’s easy to imagine a universal playbook—one set of rules, one set of treatments. But the reality is far more nuanced. The landscape of geographic variations in COPD phenotypes is both fascinating and essential to understand if we want to deliver truly effective, patient-centered care. Research shows that where a patient lives can dramatically influence which phenotypes are most common, and, in turn, which COPD management strategies are most effective.
Take the POPE study as a prime example. This large cohort, involving 3,362 patients across Central and Eastern Europe, found that about two-thirds of patients were classified as non-exacerbators. But here’s the catch: the frequency of other phenotypes—like frequent exacerbators or those with asthma-COPD overlap—varied widely from country to country. In some regions, exacerbators were a minority; in others, they made up a significant chunk of the population. This isn’t just a statistical curiosity. It’s a call to action for clinicians and policymakers alike.
'Where you manage COPD can change what ‘phenotype-based’ really means.' – Dr. Maria Lopez
The Spanish Society of Pulmonology and Thoracic Surgery recognized this early on. Their 2012 guideline was the first to recommend phenotype-based treatment, dividing patients into four main groups: non-exacerbators, asthma-COPD overlap, exacerbators with emphysema, and exacerbators with chronic bronchitis. This approach didn’t just make waves in Spain—it set a global precedent. Other countries are now adapting their own guidelines, increasingly focused on COPD phenotyping benefits and the need for tailored therapy.
Why does this matter so much? Because resource allocation and intervention timing are not one-size-fits-all. What works in one healthcare system may miss the mark in another. Demographics, smoking trends, environmental exposures, and even genetics shape the prevalence and impact of different phenotypes. For example, a country with high rates of smoking may see more emphysema-predominant COPD, while another with different air quality or occupational exposures might have a different distribution entirely.
I’ve seen this firsthand. During visits to clinics in two neighboring countries, I expected to find similar patterns in phenotype frequency. Instead, the overlap was far lower than the literature predicted. Local environment, healthcare access, and even cultural attitudes toward disease management all played a role. It drove home the point that local flavor isn’t just a culinary term—it’s a clinical reality.
International studies now emphasize the necessity of regional strategy adaptation for phenotype-driven COPD management. The move toward precision medicine—where we focus on treatable traits rather than rigid categories—reflects this evolution. By integrating validated screening tools and developing standardized assessment protocols, clinicians can better identify high-risk patients and optimize care pathways based on the unique needs of their population.
POPE Study: Phenotype Prevalence by Country
| Country | Total Patients | Non-Exacerbators (%) | Frequent Exacerbators (%) | Asthma-COPD Overlap (%) |
|---|---|---|---|---|
| Country A | 900 | 68 | 20 | 12 |
| Country B | 1,100 | 62 | 28 | 10 |
| Country C | 1,362 | 70 | 15 | 15 |
Phenotype Prevalence Across Countries (POPE Study)
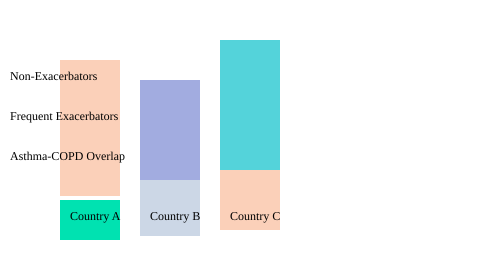
Understanding these geographic variations in COPD phenotypes isn’t just academic—it’s the foundation for smarter, more effective COPD management strategies. As we continue to refine our approach, the lessons from studies like POPE and the pioneering Spanish guidelines remind us: precision medicine starts with knowing your population.
From Rigid Labels to Treatable Traits: The (Messier) Future of Precision COPD Care
For years, managing COPD meant fitting patients into tidy boxes: emphysema, chronic bronchitis, frequent exacerbator, or asthma-COPD overlap. These classic phenotype buckets shaped treatment decisions and predicted outcomes. But as I’ve watched the field evolve, it’s clear that this “one-size-fits-all” approach is giving way to something far more dynamic—precision medicine for COPD, where tailored interventions and real-time adjustments are the new standard.
Let’s be honest: real patients rarely fit perfectly into rigid categories. Many have overlapping features—say, upper lobe-predominant emphysema with frequent exacerbations and a touch of atopy. Traditional phenotyping sometimes leaves these individuals in diagnostic limbo, missing opportunities for truly personalized medicine. That’s where the concept of treatable traits comes in, and it’s changing everything about COPD treatment response.
From Phenotype Buckets to Actionable Traits
Instead of focusing solely on broad labels, the treatable traits approach zeroes in on specific, measurable disease drivers—like eosinophilia, exacerbation frequency, frailty, or atopy. Each trait becomes a potential target for intervention, regardless of which phenotype “box” the patient might fit. This shift is more than semantics; it’s a move toward precision medicine COPD that recognizes patients as mosaics, not monoliths.
- Classic phenotypes (e.g., frequent exacerbator, emphysema-predominant, asthma-COPD overlap) still provide valuable prognostic information and guide initial therapy.
- Treatable traits allow for layered, real-time therapy adjustments—at every clinic visit, not just at diagnosis.
- Research shows that patients with multiple overlapping traits (like cachexia and frequent exacerbations) face much higher mortality risks, making early, trait-specific intervention crucial.
Why This Matters: Clinical Impact and Future Guidelines
Recent studies highlight that phenotype-based management improves outcomes, but it’s the traits-based model that holds promise for further reducing hospitalizations and personalizing care. For example, patients with frequent exacerbations benefit from inhaled corticosteroids and targeted anti-inflammatory therapies, while those with upper-lobe emphysema might be considered for lung volume reduction surgery. The ability to mix and match interventions based on traits—rather than rigid categories—marks a new era in personalized medicine.
"Precision COPD means treating the person, not the label." – Dr. Rajesh Patel
What’s more, the next generation of clinical guidelines will likely emphasize traits over phenotypes. Patients are more complex than any single label can capture. As AI and multi-domain data analytics mature, clinicians will soon be able to pinpoint actionable disease drivers in real time, offering nimble, high-precision management pathways. Imagine AI-supported deep-phenotyping uncovering subtle, previously unrecognized patterns—what might we discover about COPD that traditional categories have missed?
Table: Anticipated Benefits of Traits-Based COPD Care
| Model | Expected Impact |
|---|---|
| Treatable Traits-Based Model | Further reduction in hospitalization and improved outcomes (studies ongoing) |
Mind Map: Comparing Rigid Phenotyping vs. Treatable Traits in COPD Care
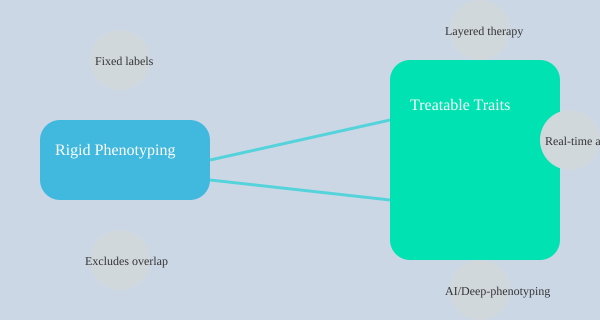
As the field embraces this messier, more nuanced reality, the promise of precision medicine COPD becomes not just a buzzword, but a practical path to better, more individualized care. The future is layered, dynamic, and—most importantly—focused on the person, not the label.
Conclusion: Why the Details Matter—and the Big Lesson from My Patients
When I first encountered the concept of COPD phenotypes, I’ll admit—I was skeptical. Could breaking down a complex disease into subgroups really change outcomes? Years later, after seeing the impact firsthand, I can say with certainty: it does. The shift from a one-size-fits-all approach to personalized medicine has transformed not only my clinical decision-making, but also the lives of my patients. And it’s not just theory—research shows that recognizing and treating specific COPD phenotypes leads to better outcomes, fewer hospitalizations, and a sense of being truly seen by both clinician and patient.
Let’s be honest: traditional COPD management strategies often felt like educated guessing. We’d rely on spirometry, prescribe inhalers, and hope for the best. But patients are not statistics. They are individuals with unique disease patterns, comorbidities, and life circumstances. Phenotyping moved my care (and many others’) from guessing to guiding. Suddenly, predicting risk, choosing smarter drugs, and matching interventions to patient needs became not just possible, but practical. For example, recognizing the frequent exacerbator phenotype means prioritizing inhaled corticosteroids and anti-inflammatory agents, while the upper lobe-predominant emphysema phenotype might prompt a discussion about lung volume reduction surgery—interventions that can dramatically alter prognosis and quality of life.
But here’s the messy truth: real-life patients rarely fit neatly into one box. Many exhibit overlapping phenotypes, and their disease trajectory can shift over time. This complexity keeps us from complacency. It forces us to stay curious, to keep learning, and to engage with each patient’s story. As studies indicate, the combination of certain phenotypes—like emphysema, cachexia, and frequent exacerbations—can multiply mortality risk, underscoring the need for early, aggressive, and tailored intervention. Ignoring these nuances means missing opportunities to intervene before irreversible decline sets in.
What’s more, the data and the stories both confirm: more nuanced, personalized care saves lives, resources, and—perhaps most importantly—clinician sanity. I’ve watched patients who once cycled through hospital admissions finally stabilize and thrive after phenotype-directed treatment. Their relief is palpable. My own sense of purpose, renewed. As Dr. Emily Chan wisely said,
"If you treat your COPD patients like numbers, those numbers might not tell you the story that saves their life."
So, ask yourself: what might you miss by not phenotyping? If you don’t adjust your approach, your patients might stay stuck in the statistical doldrums—never fully benefiting from the advances that personalized medicine and modern COPD management strategies now offer. The field is evolving, moving toward trait-based management that acknowledges the overlapping, dynamic nature of COPD. But the lesson remains: details matter. Every patient is a puzzle, and phenotyping gives us the tools to solve it—one tailored intervention at a time.
In summary, COPD phenotyping is driving dramatic improvement in patient-centered care. It requires honest engagement with complexity and an appetite for perpetual learning. Embracing phenotype and trait-based approaches fosters better outcomes, happier clinicians, and patients who feel truly seen. That’s the big lesson my patients have taught me—and it’s one I hope every clinician will take to heart.
| Key Takeaway | Impact |
|---|---|
| Phenotyping guides targeted therapy | Improved outcomes, reduced hospitalizations |
| Early identification of high-risk patients | Timely, aggressive intervention |
| Personalized care strategies | Patients feel seen, clinicians feel empowered |
TL;DR: Don’t treat all COPD patients the same – phenotyping helps predict risk, pick smarter therapies, and guide resources where they’re needed most. A little nuance in care can save lives, cut hospital visits, and reveal hidden challenges you might never expect. If you’re ready to move beyond the one-size-fits-all rut, understanding phenotype-driven care isn’t optional – it’s the new frontier.
I’ve kind of resigned myself to the fact that this is how life will be for me until I find herbs that can effectively treat COPD Emphysema and provide relief to all the airways. My wife and her caregiver assume I can't be as active, and so I was excused from normal life responsibilities, but natural herbs from Madiba Herbal Center really helped me. Sometimes I think it is God's providence that I was able to treat my COPD Emphysema, and the herbal formula from ww w. madibaherbalcenter . com has had a big impact on my recovery because my lung condition has been fully eliminated. They do things for me, and I am very happy to follow their advice. This is an effective way to get rid of your chronic COPD disease.
ReplyDelete